[最も好ましい] maximum yield chemistry formula 192278-How to calculate the maximum yield
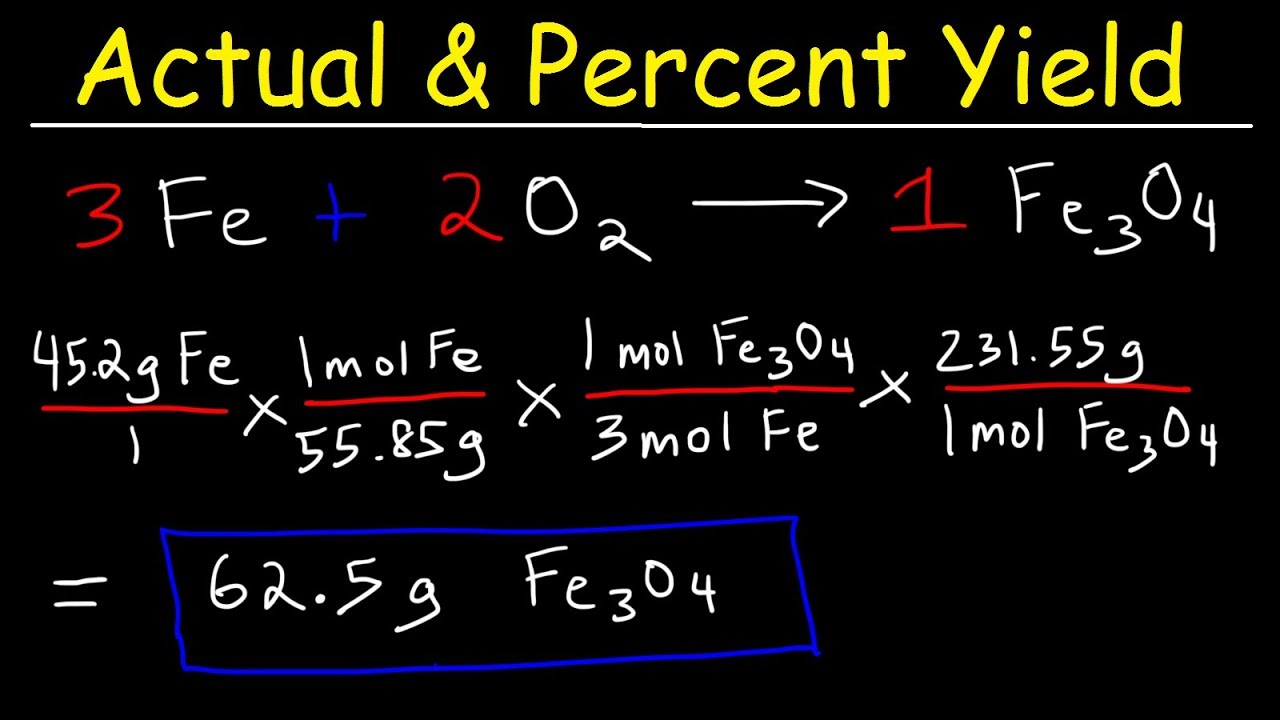
In this example, the molar mass of CO 2 is about 44 g/mol (Carbon's molar mass is ~12 g/mol and oxygen's is ~16 g/mol, so the total is 12 16 16 = 44) Multiply 04 moles CO 2 x 44 g/mol CO 2 = ~367 grams The theoretical yield of the experiment is 367 grams of CO 2The theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid) The molar yield of the product is calculated from its weight (132 g ÷ g/mol = 15 mol) The % yield is calculated from the actual molar yield and the theoretical molar yield (15 mol ÷ mol × 100% = 75%)Mass(NH3) = moles(NH3) × molar mass(NH3) predicted mass NH3= 8 × (14 3 × 1) = 8 × 17 = 136 g Theoretical yield= predicted mass = 136 g Percentage yield = (actual yield÷ theoretical yield) × 100 Substituting the vales for actual yield and theoretical yield into the equation

4 4 Reaction Yields Chemistry
How to calculate the maximum yield
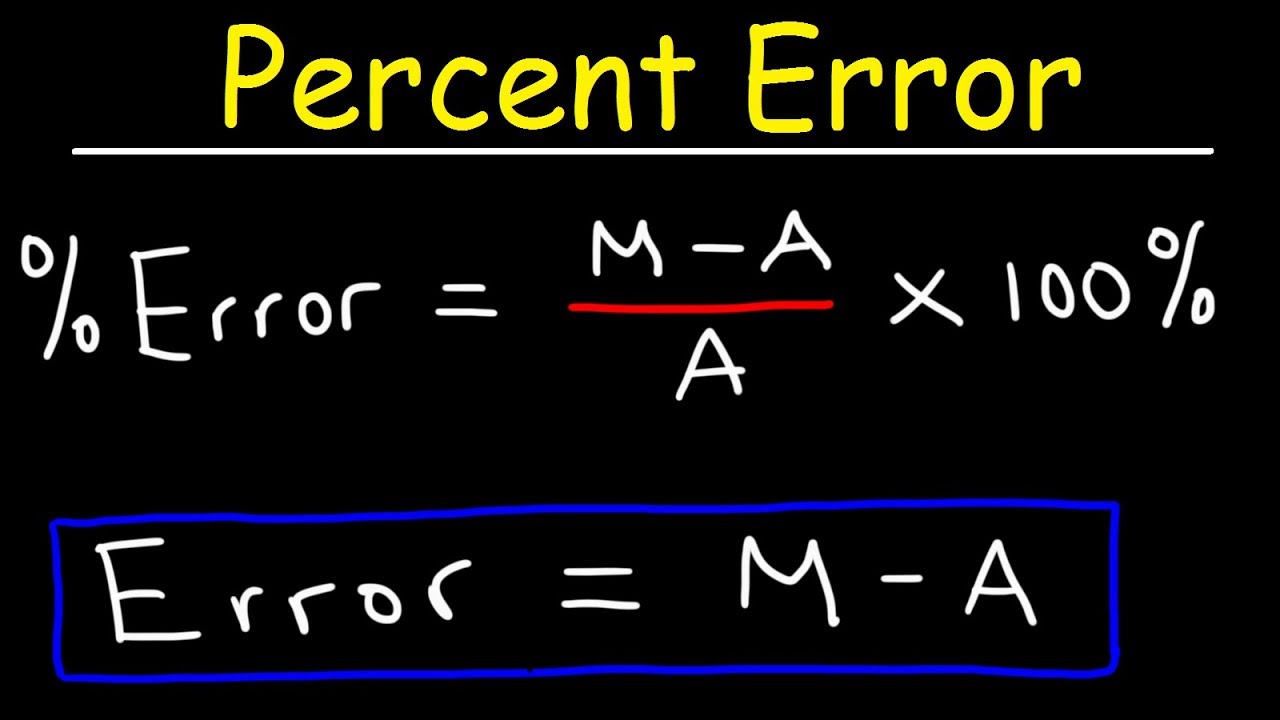
How to calculate the maximum yield-Chemists have to be concerned with just how completely their reactants react to form products To compare the amount of product obtained from a reaction with the amount that should have been obtained, they use percent yield You determine percent yield of a chemical reaction with the following formula Lovely, but what is an actualThe maximum yield of Cu = 5 x 635/795 = 399 g (2 dp, 3 sf)(b) If 391 g of copper was actually obtained, calculate the percentage yield of the reaction % yield = 100 x actual yield / theoretical maximum yield % yield = 100 x 391 / 399 % yield of Cu = 980% (3 sf, 1 dp)



Theoretical Yield Calculator
Fluorescence quantum yield is measured on a scale from 0 to 10, but is often represented as a percentage A quantum yield of 10 (100%) describes a process where each photon absorbed results in a photon emitted Substances with the largest quantum yields, such as rhodamines, display the brightest emissions;Yield Example desired product , r D =k 1 C A 2 C B undesired product , r U =k 2 C A C B To maximize the selectivity of D with respect to U run at high concentration of A and use PFRIf three or more values occur at the maximum frequency the data set is multimodal ∑x µ = mean value Σx i = sum of all data values (x 1, x 2, x 3, n = number of data values √ ∑(x ) Standard Deviation σ = standard deviation x i = individual data value ( x 1, x 2, x 3, n = number of data values Range Range = x max x min x = maximum data value x
Yield Example desired product , r D =k 1 C A 2 C B undesired product , r U =k 2 C A C B To maximize the selectivity of D with respect to U run at high concentration of A and use PFRYield is the mass of product formed in a chemical reaction Actual yield is the mass of product formed in an experiment or industrial process Theoretical yield is the mass of product predicted by the balanced chemical equation for the reaction Percentage yield = (actual yield ÷ theoretical yield) × 100Basically since the molar ratio is 11 there are 02 moles of Fe there is 02 moles of Fecl3 going back to the question it asked for the mass of FeCl3 remember you can rearrange moles=mass/mr to mass=moles x Mr 02 (moles) x 1625 (Mr of FeCl3) = 325 (mass of FeCl3) 4 reply
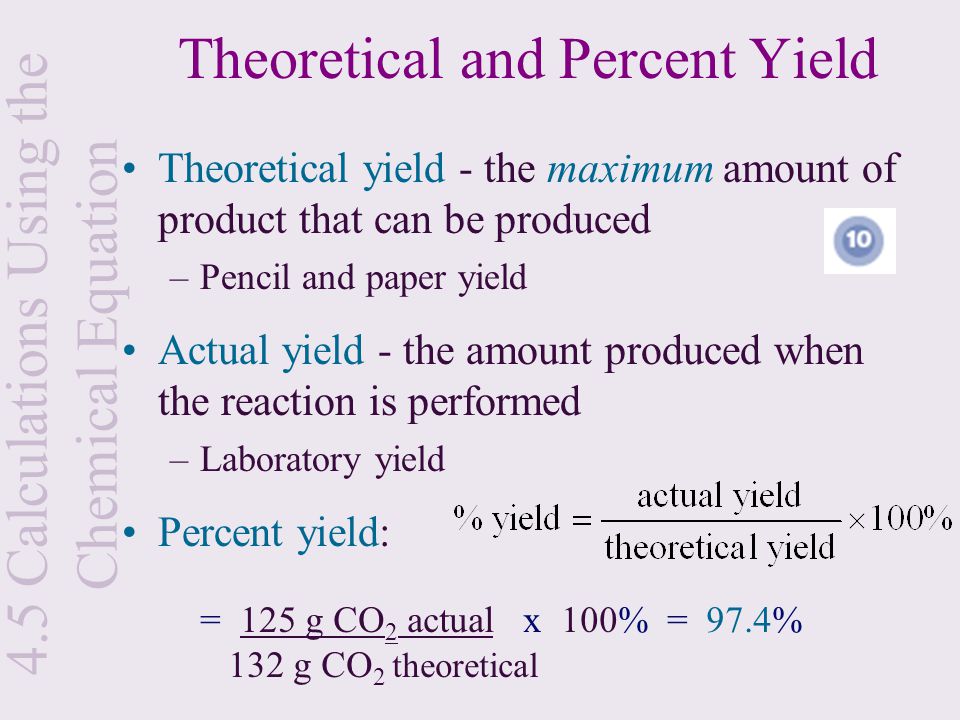
22 Gas and liquid phase chemical reactions The most complex kinetic model proposed for flue gas irradiation include over 0 chemical species contributing to more than 850 chemical reactions in the gas phase (Schmitt and Dibble, 11)Our kinetics consider, for the gas phase, 100 chemical reactions and 53 ionic, molecular and radical species selected from different sources and assembledAbout Percent Yield in the Organic Laboratory DefinitionsTheoretical Yield The maximum amount of product if ALL of the limiting reagent reacted exactly as described by the balanced equation, with no losses due to side reactions or spillage Percent Yield The amount of purified product obtained, divided by the theoretical yieldThe percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage (381) Percent Yield = Actual Yield Theoretical Yield × 100 % Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical production
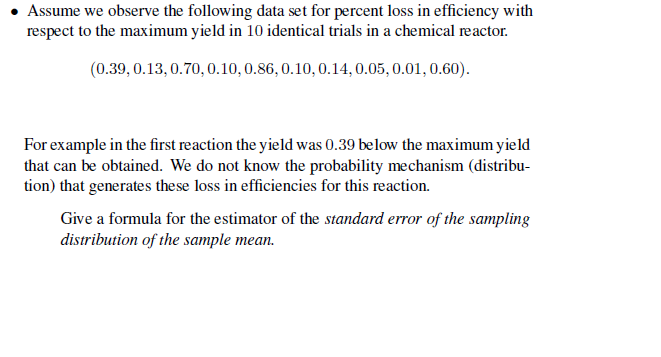


Solved Assume We Observe The Following Data Set For Per Chegg Com


Stoichiometry Chemistry Libretexts
Science AP®︎/College Chemistry beta Chemical reactions Stoichiometry Stoichiometry Stoichiometry Worked example Calculating amounts of reactants and products Limiting reactant and reaction yields This is the currently selected itemThis may seem confusing to a lot of people which is why it's better to use the percent yield calculator to perform the calculations Still, if you want to do the calculation by hand, you should use the percent yield equation percent yield = (experimental mass of the desired product / theoretical mass of the desired product) * 100However, compounds with quantum yields of 010 are still considered quite fluorescent



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com



Solved 1 Write A Reaction Using An Alkene And An Acid To Chegg Com
Question 1 During a chemical reaction 06 g of product is made The maximum calculated yield is 14 g What is the percentage yield of this reaction?Calculate the maximum theoretical yield of calcium oxide that can be produced from 250 g of calcium carbonate Write down the balanced chemical equation CaCO 3 \(\rightarrow\) CaO CO 2It is the theoretical yield However, it is impossible for a ration to give 100 percent yield Because there is always some loss of reactant due to different factors Formula You can also find the theoretical yield yourself using the theoretical yield formula if percentage yield or actual yield is known Theoretical yield = Actual yield 100



Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry
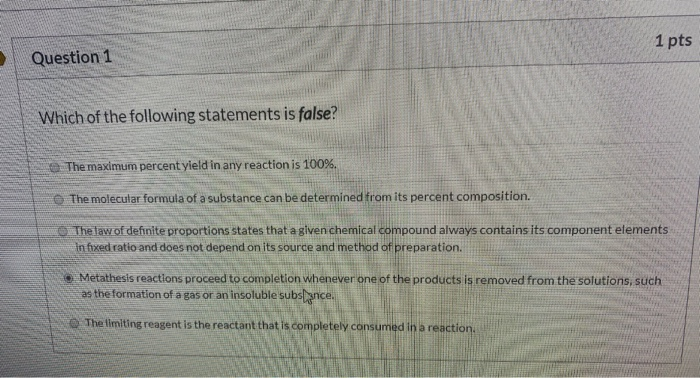


Solved 1 Pts Question 1 Which Of The Following Statements Chegg Com
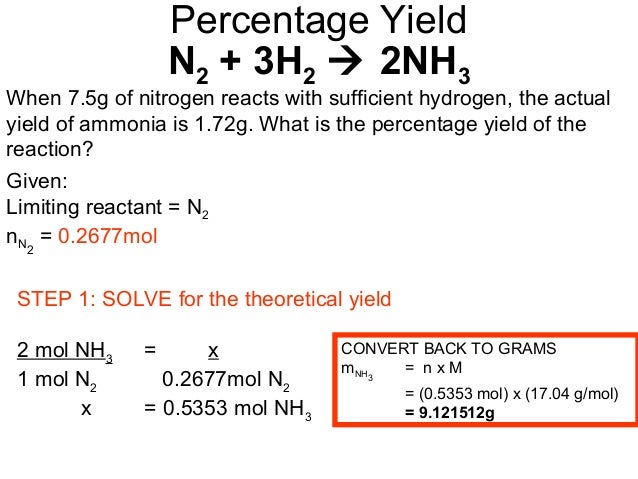
The question reads that the actual yield from 100 grams of Calcium Hydroxide is 50 grams of Calcium Oxide Hence, the percent yield is given by Percent Yield = Actual Yield/Theoretical Yield xTheoretical yield (g) =(moles of LR)• moles of P moles of LR = 296 g Percent yield = 243 g 296 g •100 / 04 = 92% Calculate Percent Yield Since not all of the crude product was recrystallized, we need to account for that in the calculation of percent yield In other words, the percent yield should reflect what you would have gotten if youFrom the previous worked example we found the maximum mass of ammonium sulfate that could be produced The theoretical yield (or maximum mass) of ammonium sulfate that can be produced is 2,69 kg Calculate the percentage yield % yield = actual yield theoretical yield × 100 = 2,5 2,694 (100) = 92,8 %



Question Video Calculating The Percentage Yield Of The Recreation Of Aqueous Copper Sulfate With Zinc Metal Nagwa



Theoretical Actual Percent Yield Error Limiting Reagent And Excess Reactant That Remains Youtube
In a chemical reaction the maximum amount of product formed is determined by the amount of limiting reactant that is used up Stoichiometry is used to predict this amount of product It is known as the theoretical yield Theoretical Yield Formula Questions 1 Determine the theoretical yield of H 2 O (in moles) in the following reaction,The theoretical yield is the maximum possible mass of a product that can be made in a chemical reaction It can be calculated from the balanced chemical equationScience AP®︎/College Chemistry beta Chemical reactions Stoichiometry Stoichiometry Stoichiometry Worked example Calculating amounts of reactants and products Limiting reactant and reaction yields This is the currently selected item
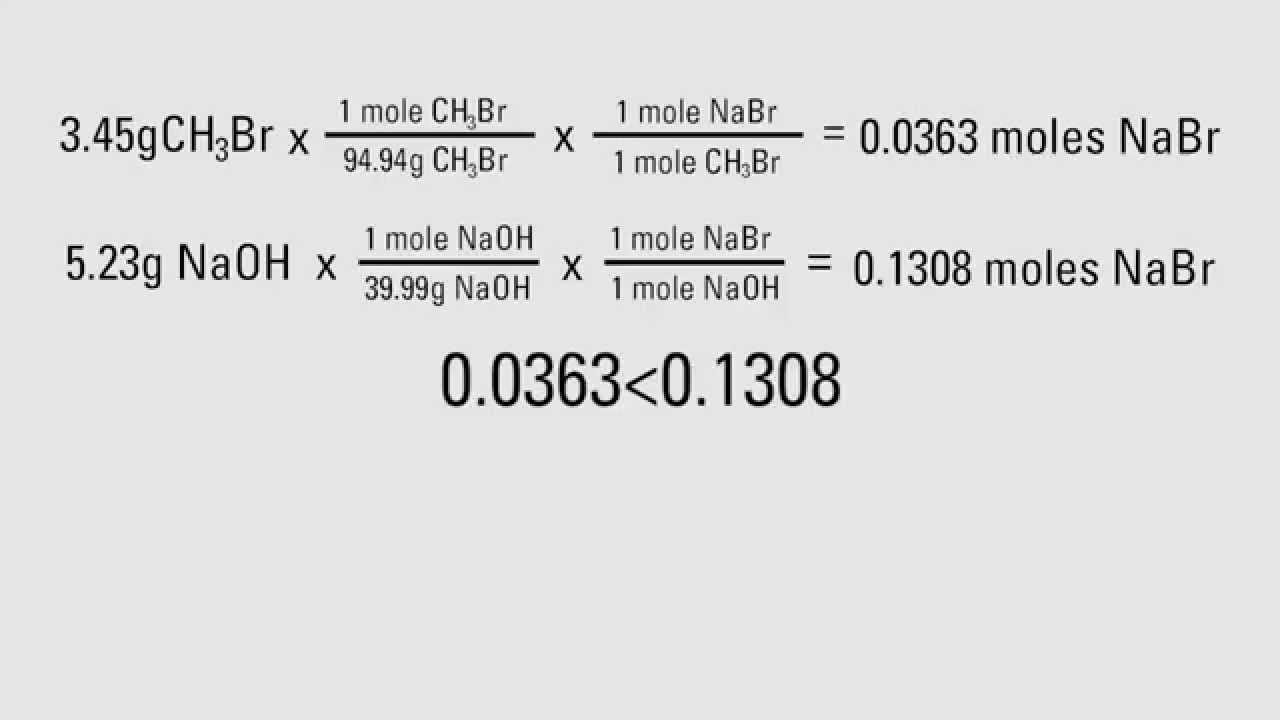


How To Calculate Theoretical Yields Youtube



Stoichiometry Product Yield And Limiting Reactants Protocol
Find my revision workbooks here https//wwwfreesciencelessonscouk/workbooksIn this video, which is the first of two, we look at how to calculate the percThe extent to which a reaction's theoretical yield is achieved is commonly expressed as its percent yield percent yield = actual yield theoretical yield ×100% percent yield = actual yield theoretical yield × 100 %The formula for calculating the percent yield is Percentage yield = mass of actual yield ÷ mass of theoretical yield × 100% Let's assume that you obtained an actual yield of 850 grams Then, the percent yield would be Percentage yield of NaCl = 850 grams ÷ 993 grams × 100% Percentage yield of NaCl = 8559%
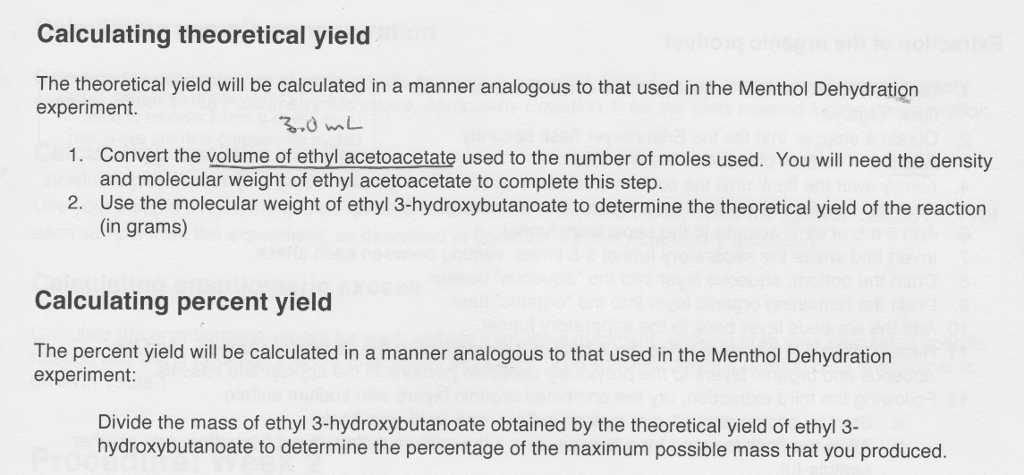


How To Calculate Percentage Yield Formula How To Wiki



Stoichiometry
Find my revision workbooks here https//wwwfreesciencelessonscouk/workbooksIn this video, which is the first of two, we look at how to calculate the percYield}$$\times$ 100% Percentage yield = $\frac{06}{14}$$\times$ 100%In a chemical reaction the maximum amount of product formed is determined by the amount of limiting reactant that is used up Stoichiometry is used to predict this amount of product It is known as the theoretical yield Theoretical Yield Formula Questions 1 Determine the theoretical yield of H 2 O (in moles) in the following reaction,


Q Tbn And9gcsbwel4mzamey6ite9bp 08j Tpx0zptf7ruyvck4xodffyxfeu Usqp Cau



Stoichiometry Review Ppt Download
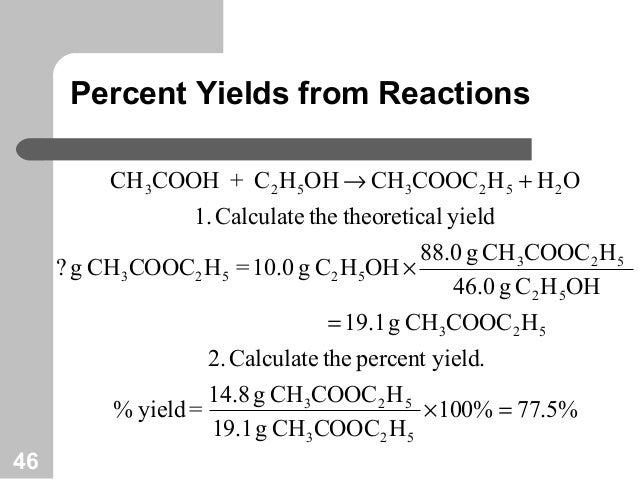
The extent to which a reaction's theoretical yield is achieved is commonly expressed as its percent yield percent yield = actual yield theoretical yield ×100% percent yield = actual yield theoretical yield × 100 %Percent yield = actual yield / theoretical yield x 100% percent yield = 15 g / 19 g x 100% percent yield = 79% Usually, you have to calculate the theoretical yield based on the balanced equation In this equation, the reactant and the product have a 11 mole ratio, so if you know the amount of reactant, you know the theoretical yield is the same value in moles (not grams!)In reality, most reactions are not perfectly efficient If you perform the experiment, you'll end up with a smaller amount, the actual yield To express the efficiency of a reaction, you can calculate the percent yield using this formula %yield = (actual yield/theoretical yield) x 100 A percent yield of 90% means the reaction was 90% efficient, and 10% of the materials were wasted (they failed to react, or their products were not captured)
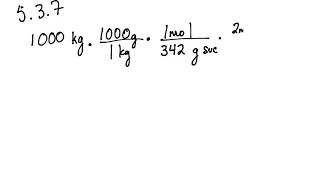


5 3 Calculating Reaction Yields Problems Chemistry Libretexts


The Stoichiometry Of Product Formation And Percent Yield
Find my revision workbooks here https//wwwfreesciencelessonscouk/workbooksIn this video, which is the second of two, we continue to look at how to calcuThe maximum possible yield is the amount of product you would get if 100% of the limiting reactant was used up in the reaction It's a theoretical calculation because no reaction converts reactantsUsing the theoretical yield equation above, we know our grams of hydrogen will yield x 1/2 x 1/5 x 250 = 500 grams of product How to Calculate Theoretical Yield in Chemistry The individual steps of the process of calculating theoretical yield looks like this
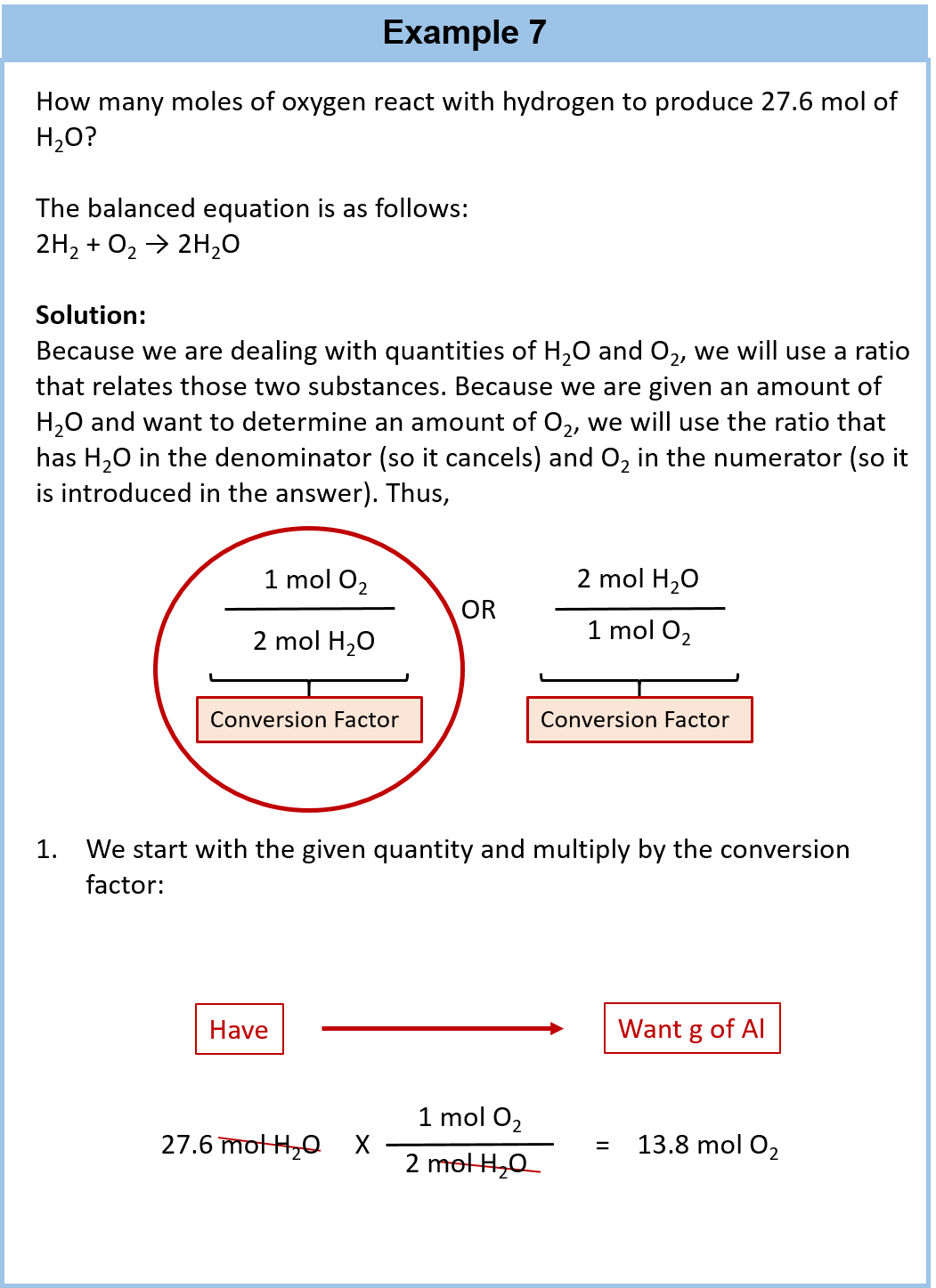


Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry



Reaction Stoichiometry Boundless Chemistry
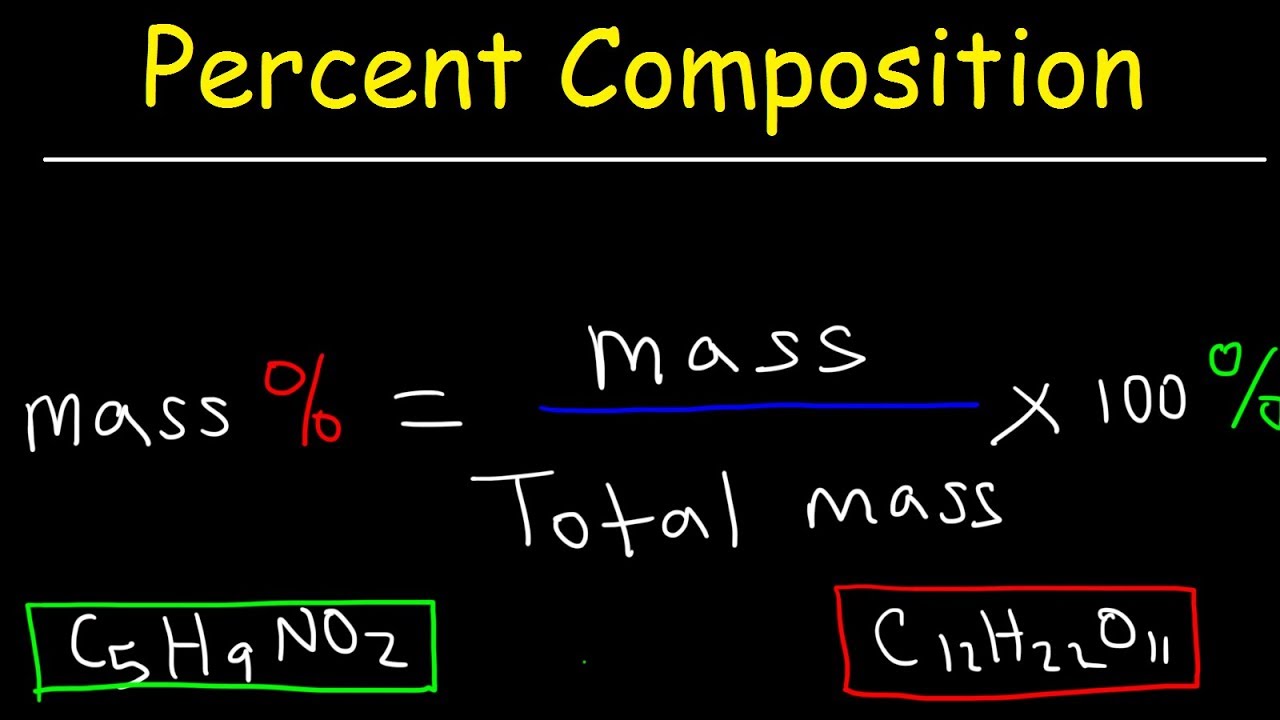
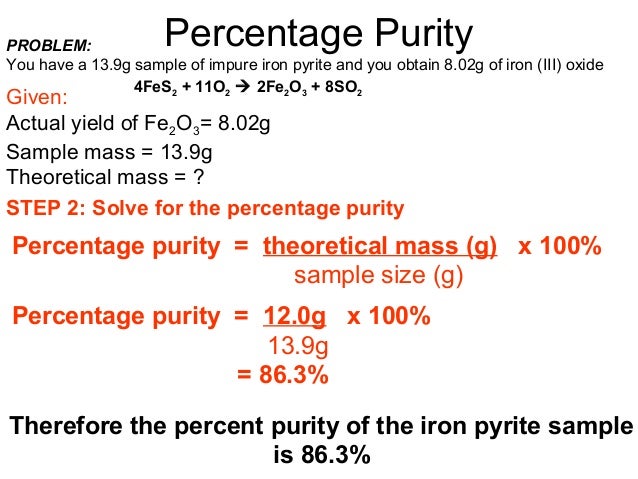
Thus, the percentage yield is \(\mathrm{\%\ yield =\dfrac{61\ tons}{96\ tons}\times 100 = 64 \%}\) Due to chemical equilibrium or the mass action law, the limiting reagent may not be completely consumed Thus, a lower yield is expected in some cases Losses during the recovery process of the product will cause an even lower actual yieldThe theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid) The molar yield of the product is calculated from its weight (132 g ÷ g/mol = 15 mol) The % yield is calculated from the actual molar yield and the theoretical molar yield (15 mol ÷ mol × 100% = 75%)So, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)


Q Tbn And9gctje7pkewyqa0nnrn06zpgzepy Yorrmegghf1btx8a49kkxzp8 Usqp Cau



Theoretical Yield Calculator
The maximum possible yield is the amount of product you would get if 100% of the limiting reactant was used up in the reaction It's a theoretical calculation because no reaction converts reactants to products with perfect efficiency For example, zinc metal reacts with iodine gas to form zinc iodide Zn(s) I2(g) > ZnI2(s)So, to stop you from wondering how to find theoretical yield, here is the theoretical yield formula mass of product = molecular weight of product * (moles of limiting reagent in reaction * stoichiometry of product)Solution Substitute the values in the corresponding formula Percentage yield = $\frac{Actual\;


Http Cdochemistrychristman Pbworks Com W File Fetch Ap chemistry unit 1 13 14 87 answers Pdf


18 Percentage Yield
1) Increase the temperature 2) Increase the concentration of reactants One to one online tution can be a great way to brush up on your Chemistry knowledgeHave a Free Meeting with one of our hand picked tutors from the UK's top universitiesIn print and online, Maximum Yield is your source for cannabis and cultivation knowledge, with informative grow articles and tips to enhance your life with mmj You must be 19 years of age or older to enter this siteHow can you increase the yield of product from a reaction?



4 4 Reaction Yields Chemistry


How To Calculate Theoretical Yield And Percent Yield Youtube
The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage (1291) Percent Yield = Actual Yield Theoretical Yield × 100 % Percent yield is very important in the manufacture of products Much time and money is spent improving the percent yield for chemical productionWhat is the theoretical yield in grams for this reaction?% yield = actual amount of desired chemical obtained x 100 / maximum theoretical amount that could be formed If the reaction doesn't work the yield is zero or 0% If the reaction works perfectly and you obtain all the product, the yield is 100%, BUT this never happens in reality (as already discussed above)
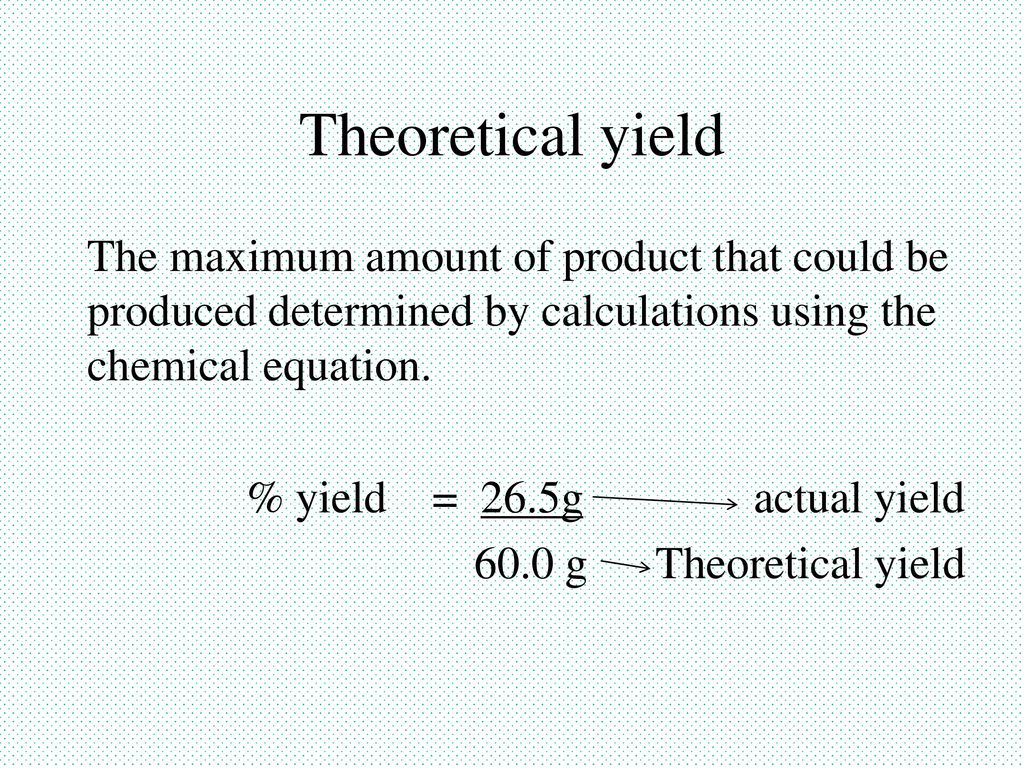


Yield Amount Of Products Ppt Download
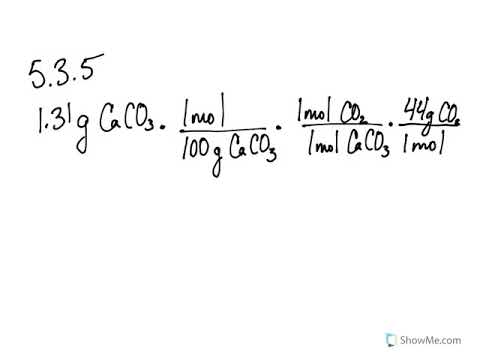


5 3 Calculating Reaction Yields Problems Chemistry Libretexts
The reaction yield (absolute yield) of a chemical reaction is the amount of pure and dry product yielded in a reaction Normally, in order to measure the efficiency of a chemical reaction in organic synthesis, the relative or percentage yield (%) is calculated Before calculating the yield of a reaction (necessary when preparing the laboratory notebook) it is crucial to know the stoichiometryThe question reads that the actual yield from 100 grams of Calcium Hydroxide is 50 grams of Calcium Oxide Hence, the percent yield is given by Percent Yield = Actual Yield/Theoretical Yield xArithmetic Mean Geometric Mean Quadratic Mean Median Mode Order Minimum Maximum Probability MidRange Range Standard Deviation Variance Lower Quartile Upper Quartile Interquartile Range Midhinge Physics Mechanics Chemistry Calculate chemical reactions and chemical properties stepbystep



Solved Questions Section I Background 1 What Is The Che Chegg Com
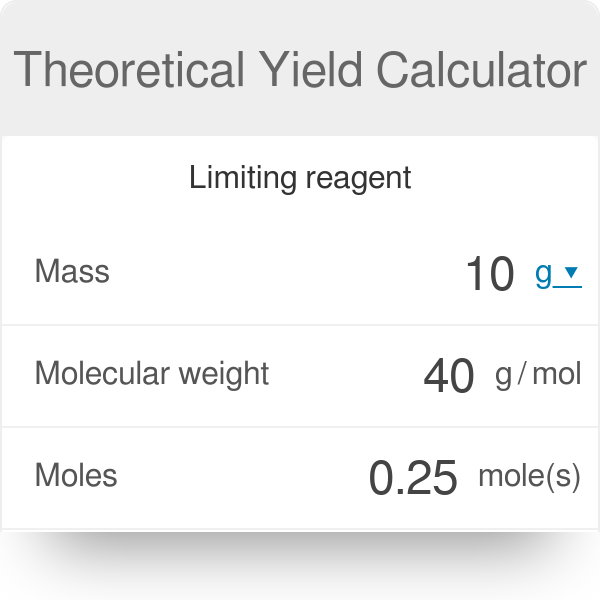


Theoretical Yield Calculator
Yield Calculations Chemistry Tutorial Key Concepts Yield is the mass of product formed in a chemical reaction Actual yield is the mass of product formed in an experiment or industrial process Theoretical yield is the mass of product predicted by the balanced chemical equation for the reactionMaximum feed rate (lbs / day) 7 Water added, gal = hypo (gal) x hypo (%) hypo (gal) x desired hypo (%) desired hypo (%) 8 Chemical feed = Chemical conc x vol pumped time pumped 9 Feed rate (lbs / day) = chemical, lbs / day (chemical, lbs) / (lb of commercial chemical) 10 ion purity (%) = (molecular weight of ion in compound) (100%)Takeaway As the groundup seed husk tissue from the coconut palm tree, coco coir has become a popular and effective growing medium for indoor and outdoor gardeners Our horticultural chemistry expert Andrew Schell has the details on what exactly coco coir is made of and how to best grow with it For starters, using a cocospecific base nutrient is the key to successful gardening with this medium



Percentage Yield Lab Answers Schoolworkhelper



Quantitative Chemistry Secondary Science 4 All
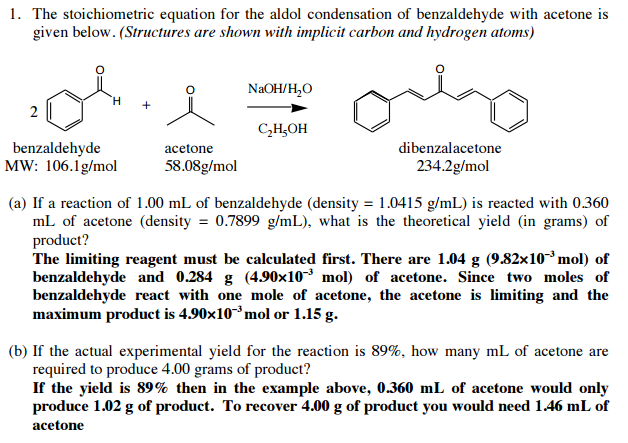


Solved If The Actual Experimental Yield For The Reaction Chegg Com



Theoretical Actual And Percent Yield Problems Chemistry Tutorial Youtube


How To Calculate Theoretical Yield And Percent Yield Youtube



Percent Yield Percent Purity Solutions Examples Videos


How To Calculate The Percent Yield And Theoretical Yield Youtube



Solution Magnesium Oxide Can Be Made By H Clutch Prep
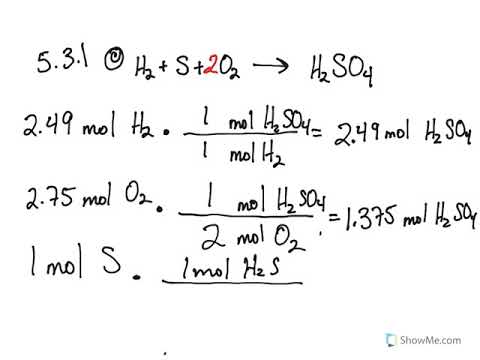


5 3 Calculating Reaction Yields Problems Chemistry Libretexts



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper


Www Assignmentexpert Com Homework Answers Chemistry Answer Pdf


18 Percentage Yield
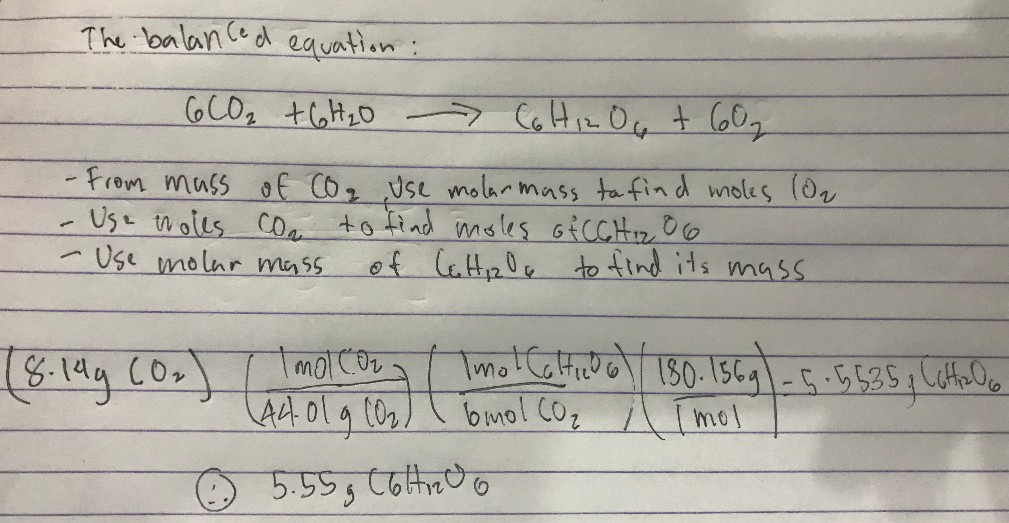


Stoichiometry Chemistry Video Clutch Prep



Theoretical Yield Of Aspirin Youtube


Elements Of Chemical Reaction Engineering



How To Calculate Percentage Yield Of Product How To Wiki



Percent Yield Chemistry Video Clutch Prep



Gcse Science Revision Chemistry Calculating Percentage Yield 1 Triple Youtube
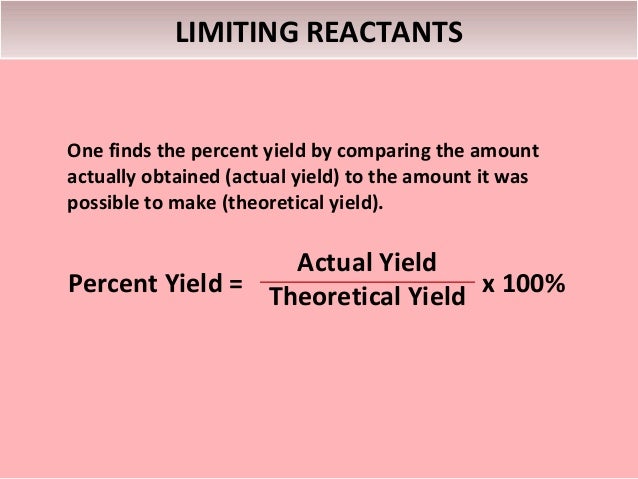


Calculations With Chemical Formulas And Equation



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com
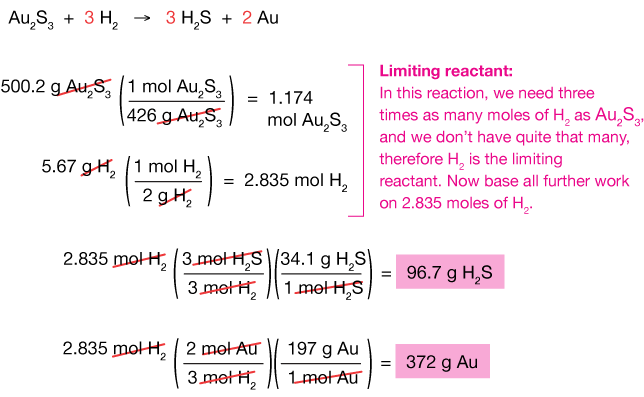


Stoichiometry


Ssl Uh Edu Chem1p C3 C3f99 Pdf



Ra9 F5hccavdqm



Calculating Percent Recovery Percent Yield



Howto How To Find Percent Yield Without Actual Yield



Stoichiometry



Stoichiometry
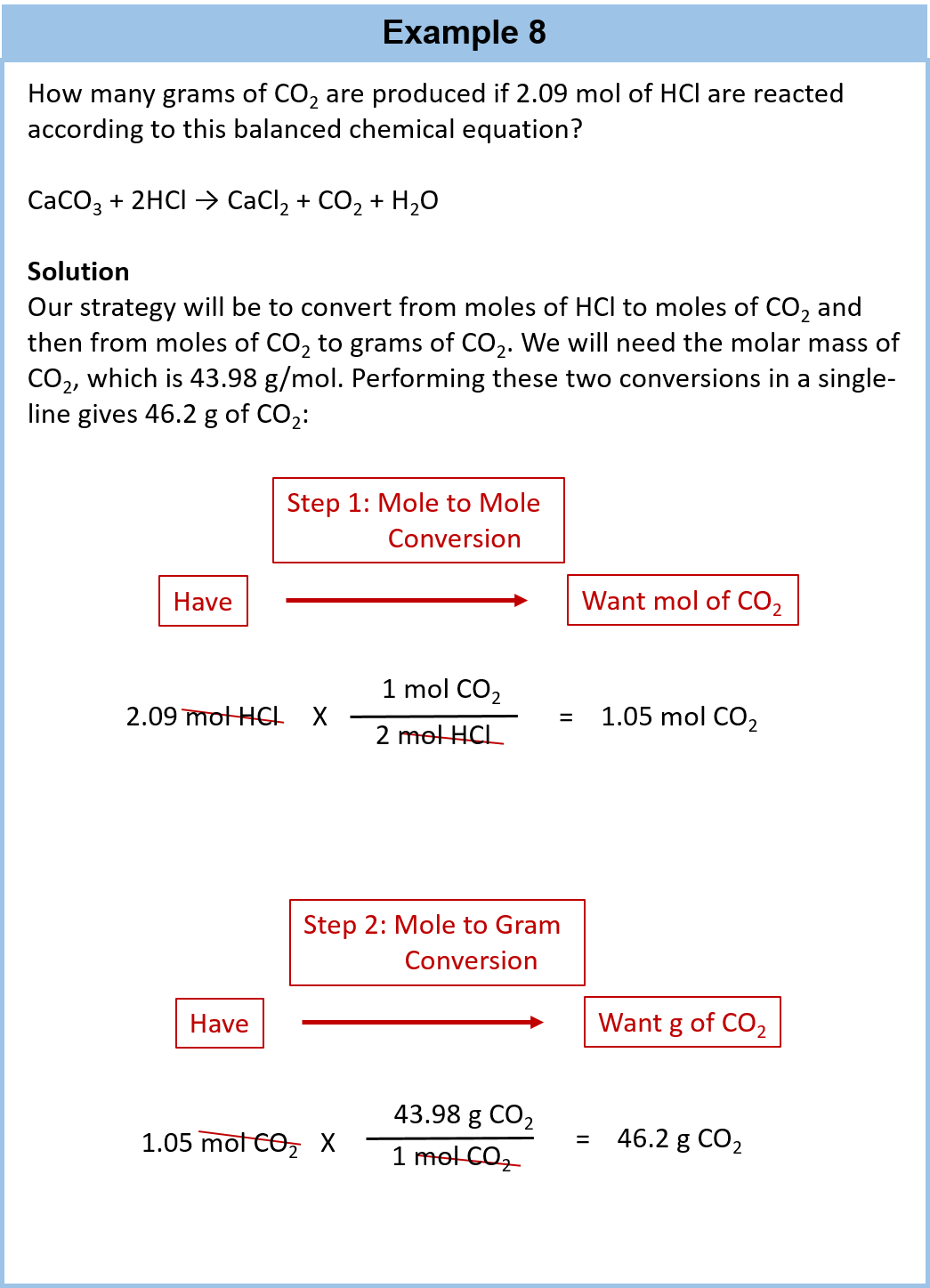


Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry



Reaction Stoichiometry Boundless Chemistry


How To Calculate Percentage Yield In Chemistry How To Wiki



Reaction Yield An Overview Sciencedirect Topics
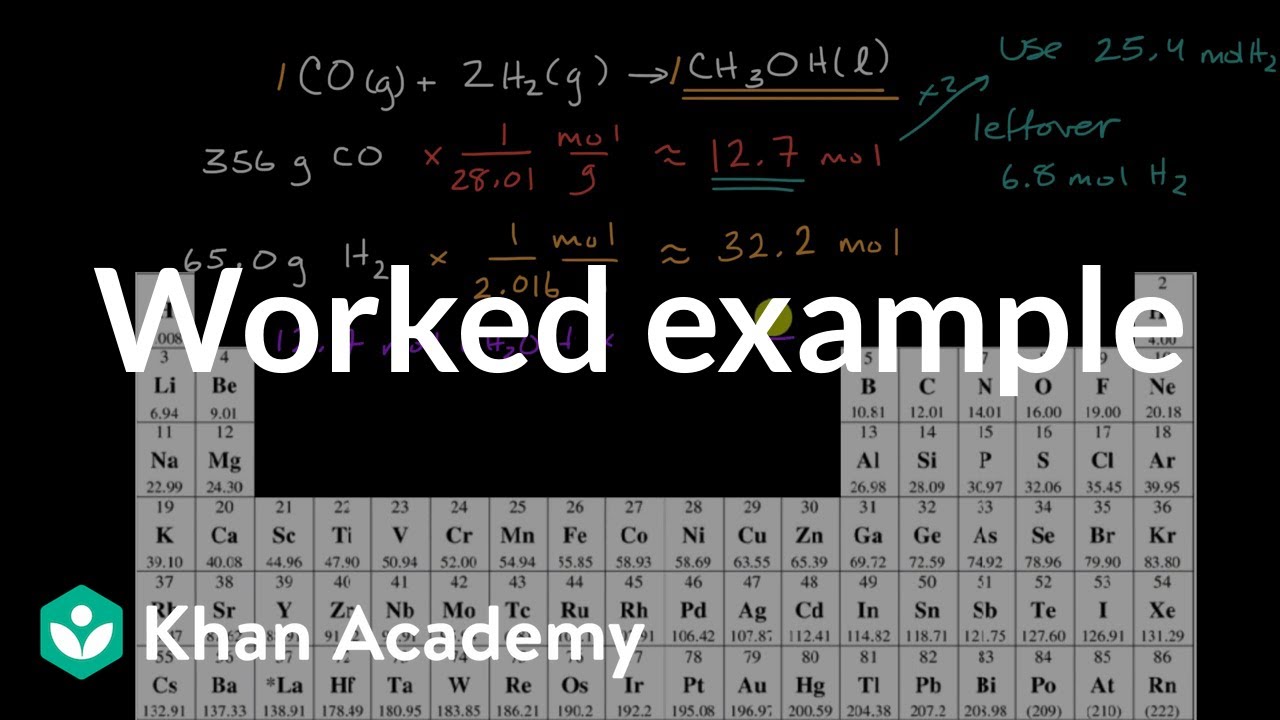


Calculating The Amount Of Product Formed From A Limiting Reactant Worked Example Video Khan Academy


Theoretical Yield Calculator



Magnesium Oxide Percent Yield Lab Report Schoolworkhelper



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Solved Mole And Percent Yield Of Reaction The Theoretical Chegg Com



Ch104 Chapter 6 Quantities In Chemical Reactions Chemistry



1 1 Write Correct Chemical Formulas Know Types Of Reactions And Write A Balanced Equation 2 A Convert G L Or Or Particles To Mole And B Use Mole Ppt Download



Yield Calculations Faculty Staff Sites


Secure Media Collegeboard Org Digitalservices Pdf Ap Apcentral Ap15 Chemistry Q2 Pdf



Calculation Methods Of Yield Strength And Ultimate Tensile Strength By Download Scientific Diagram


Chemical Equations And Reaction Stoichiometry



Lab Report On Synthesis Of Alum Using Aluminum



Chemistry Chapter 6 Flashcards Quizlet



How To Calculate Percent Yield In Chemistry 15 Steps



Yield Calculations Faculty Staff Sites



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Www Unf Edu Michael Lufaso Chem45 Chapter3 Pdf



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com



Calculations What You Need To Know Relative Formula Mass Ppt Video Online Download



Theoretical Yield Youtube


Www Gusd Net Cms Lib Ca Centricity Domain 12 Notes chapter 12 stoichiometry editted 17 Pdf



How To Calculate Theoretical Yield 12 Steps With Pictures


8 6 Limiting Reactant Theoretical Yield And Percent Yield From Initial Masses Of Reactants Chemistry Libretexts


Q Tbn And9gctx2unihrwvsl45ij5h Bp2grxrgaokdparonvakfmrfmh9cyz4 Usqp Cau



C2 Chemistry Powerpoint Presentation Free Online Download Ppt Aertax


Chapter 6 Summary Notes



Chapter 11 Combustion Updated 5 31 10



Percent Yield Chemistry Video Clutch Prep



How To Calculate Theoretical Yield And Percent Yield Youtube


Calculating Percentage Yield



Chemistry 12 3 Limiting Reagent And Percent Yield Ppt Download


Yield Calc



Actual Vs Theoretical Yield Definitions Formulas Chemistry Class Study Com



Limiting Reactant And Reaction Yields Article Khan Academy



Percentage Yield Of The Product Of A Reaction



Aleks Percent Yield Of Chemical Reactions Youtube



Stoichiometry


Www Gusd Net Cms Lib Ca Centricity Domain 12 Act 9 2 percent yield Pdf



Stoichiometry Chemistry Video Clutch Prep
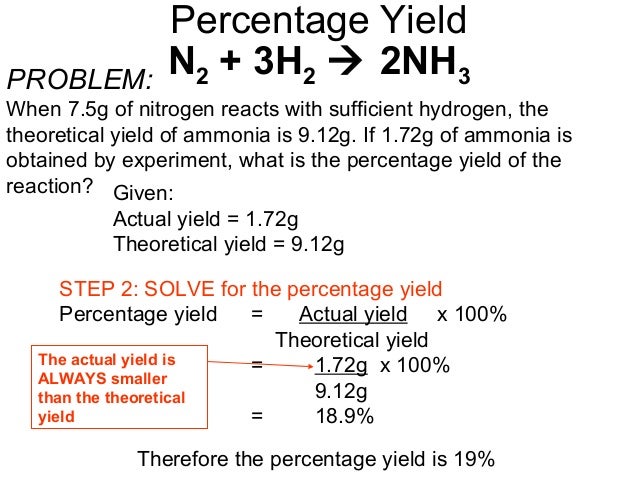


18 Percentage Yield


Q Tbn And9gcsbwel4mzamey6ite9bp 08j Tpx0zptf7ruyvck4xodffyxfeu Usqp Cau



Yield Calculations Faculty Staff Sites
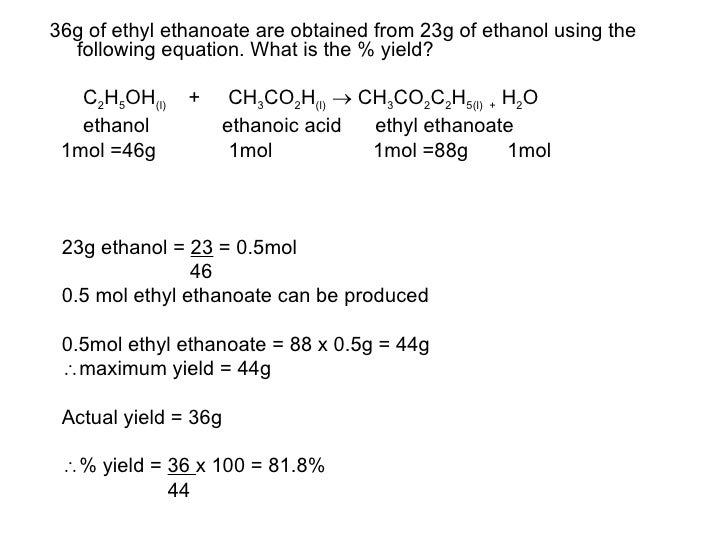


8 More On The Mole


コメント
コメントを投稿